Tiger Algebra Calculator
Finding a molecular mass
Molecular Mass
Molecular mass (also called molecular weight, the atomic weight of a molecule, formula mass, and formula weight) refers to the combined atomic weights (also called standard atomic weights) of the atoms that form a molecule. It can be found by multiplying the number of atoms of each element by their atomic weights and adding the results together.For example, contains two Hydrogen atoms, each with an atomic weight of 1.008u, and one Oxygen atom with an atomic weight of 15.999u. To find the molecular mass of , we multiply Hydrogen's atomic weights by 2, because there are two Hydrogen atoms in and we need to find their combined mass, and add it to the atomic weight of Oxygen, because there is only one Oxygen atom in , to get 18.015u.
Molecular mass is measured in unified atomic mass units, which are abbreviated as u. It is also not uncommon to see these written as amu instead, as this was the former abbreviation for unified atomic mass units. Molecular mass can also be measured in Daltons, which are often abbreviated as Da.
Relevant terms:
Atomic number
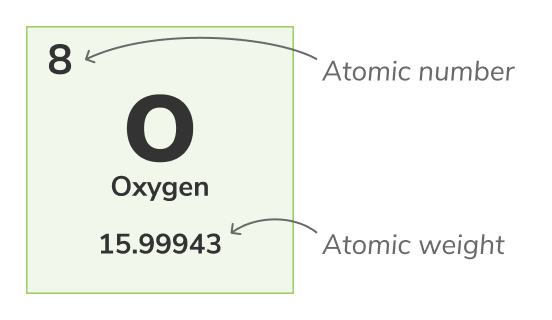
Atomic number - (also called proton number) the number of protons found in the nucleus of every atom of a chemical element. It uniquely identifies a chemical element, for example, all atoms of Oxygen have 8 protons. The atomic number is usually shown above the elements in the periodic table.Mass number
Mass number - (also called atomic mass number or nucleon number) refers to the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. One element can have different types of isotopes. For example, Oxygen can have a mass number of or . All it's isotopes have protons but different number of neutrons, or respectively.Atomic mass
Atomic mass or - is the mass of an atom. the numeric value of the atomic mass of an atom is nearly the same as its mass number value. For example, the mass of Oxygen- is 15.99491461956 u.Atomic weight
Atomic weight - (also called standard atomic weight) is the average weight of all an atom's natural isotopes. Because isotopes vary in weight, standard atomic weight is calculated by taking the average of the masses of all of an atom's isotopes. For example, Oxygen (O-16) atoms are 99.762% of all Oxygen atoms, Oxygen (O-17) atoms are 0.038% of all Oxygen atoms and Oxygen (O-18) atoms are 0.2% of all Oxygen atoms. The atomic weight of Oxygen is the average of its 3 isotopes and is equal to 15.99943 uThe atomic weight is represented by the lower number in the periodic table.